Background: Gain of function mutations of the PTPN11 gene encoding SHP2 tyrosine phosphtase are commonly seen in juvenile myelomonocytic leukemia and rarely observed in myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). In AML, PTPN11 mutation (mt) is associated with poor outcomes (Alfayez et al. Leukemia 2020). However, PTPN11 is not well characterized in other myeloid malignancies.
Patients and Methods: Between 2013 and 2019, all pts with myeloid malignancies and PTPN11 mt at Moffitt Cancer Center were identified with clinical variables obtained at time of diagnosis. Newly diagnosed and previously treated patients (pts) were included. Overall survival was calculated from time of PTPN11 detection by next generation sequencing.
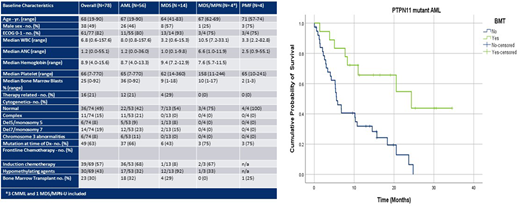
Results: We identified 78 PTPN11-mt pts, AML (56), MDS (14), MDS/MPN (4) and PMF (4). The majority (63%) of PTPN11-mt were detected at time of diagnosis. Median age was 68 years. Cytogenetics included normal (49%), complex (15%), chr. 7(19%) and chr. 5 (8%) abnormalities. Co-mutations observed in >10% were DNMT3A, NPM1, TET2, ASXL1, RUNX1, BCOR, FLT3 ITD, U2AF1, NRAS and SRSF2. In multivariate analysis including age, karyotype category and allogeneic hematopoietic stem cell transplant (AHSCT), U2AF1 was an independent covariate for inferior survival (HR 3.9 p=0.001). In newly diagnosed PTPN11-mt pts, PTPN11 VAF >20% was associated with worse outcomes (OS 8.9 vs 20.5mo p=0.043). AHSCT, which occurred 30% of pts, (n=23) was associated with improved OS (24.4 vs 5.5mo p<0.001).
WWe then compared PTPN11-mt to wild type (wt) pts among each disease category. In PTPN11-mt AML (n=56 vs n=380 in wt AML), there was female predominance (54% vs 39% p=0.04), platelet count was higher (65 vs 45 p=0.02) and no PTPN11-mt had favorable cytogenetics (0 vs 9% p=0.02). NPM1, NRAS and SRSF2 were more prevalent in PTPN11-mt AML (p<0.05). There was no difference in ORR and CR rates between mt and wt patients when comparing induction chemotherapy or hypomethylating agent. Among PTPN11-mt AML pts 32% underwent AHSCT vs 26% in wt (p=0.34). PTPN11-mt AML pts who underwent AHSCT had an OS of 24.4 vs 42.7 mo. (p=0.025) whereas OS in pts that did not undergo AHSCT was 5.6 vs 10.1 mo. (p=0.04). In our cohort, AHSCT significantly improved outcomes for PTPN11-mt AML pts (OS 5.6 vs 24.4 p=0.001). Of the 13 pts that underwent AHSCT in PTPN11-mt AML, 92.3% (n=12) received induction chemotherapy. Finally, in multivariate analysis including age, ECOG, sAML, ELN and AHSCT, PTPN11 was an independent predictor of poor outcomes (HR 1.58 p=0.015).
WNext, we evaluated pts with PTPN11-mt (n=14) vs wt (n=106) MDS. PTPN11-mt MDS pts had higher bone marrow blast % (9 vs 3 p=0.026) and more pts with MDS-EB1/2 by WHO (11/14 (79%) vs 49/105 (46%) p=.043). Interesting however, no PTPN11-mt MDS pts had complex karyotype and there was no difference in IPSS-R categories between groups. ASXL1, RUNX1 and SRSF2 were more frequently co-mutated in PTPN11-mt MDS (p<0.05) however no pts had monocyte % >10. In newly diagnosed cohort, there were no responses to frontline HMA (ORR 0/5 (0%) for mt vs 16/34(47%) wt p=0.066). Additionally, PTPN11-mt MDS had significantly worse outcomes (OS 8.7 vs 22.3 mo. p=0.001). In multivariate analysis include age, IPSS-R and AHSCT, the survival disadvantage was maintained (HR 2.9 p=0.018).
WFinally, although rare, we evaluated pts with PTPN11-mt MDS/MPN and PMF. We first compared the 4 PTPN11-mt MDS/MPN (3 CMML, 1 MDS/MPN-U) pts to a cohort of 62 PTPN11-wt MDS/MPN (53 CMML). Clinical characteristics were similar between the two cohorts with the exception of increased blast % (10 vs 4) and lower baseline hemoglobin (7.6 vs 10.9) neither of which was significant. Median time to AML transformation was 1.7 mo. in PTPN11-mt. PTPN11-mt MDS/MPN had significantly worse outcomes compared to wild type (OS 2.2 vs 15.5 mo. p=0.001). We then compared 4 PTPN11-mt PMF pts with 49 wt. There were no clinical or mutational differences between cohorts. When evaluating newly diagnosed PMF, PTPN11-mt appeared to predict worse outcomes (OS 3.4 vs 12.3 p=.063) however this was not statistically significant due to sample size.
Conclusions: The overall outcome of PTPN11-mt patients appear dismal across myeloid malignancies. In patients that are eligible, high intensity therapy followed by BMT resulted in the best outcome in PTPN11-mt AML. In conclusion, novel therapies are needed to target this high-risk subtype of myeloid malignancies.
Padron:Kura: Research Funding; Incyte: Research Funding; BMS: Research Funding; Novartis: Honoraria. Kuykendall:Novartis: Research Funding; Incyte: Research Funding; BMS: Research Funding; Blueprint Medicines: Research Funding. Lancet:Astellas Pharma: Consultancy; Celgene: Consultancy, Research Funding; Daiichi Sankyo: Consultancy; ElevateBio Management: Consultancy; Jazz Pharmaceuticals: Consultancy; Pfizer: Consultancy; Abbvie: Consultancy; Agios Pharmaceuticals: Consultancy, Honoraria. Sweet:Agios: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees; Astellas: Honoraria; BMS: Membership on an entity's Board of Directors or advisory committees; Stemline: Honoraria. Sallman:Agios, Bristol Myers Squibb, Celyad Oncology, Incyte, Intellia Therapeutics, Kite Pharma, Novartis, Syndax: Consultancy; Celgene, Jazz Pharma: Research Funding. Komrokji:Novartis: Honoraria; Acceleron: Honoraria; Incyte: Honoraria; Abbvie: Honoraria; Geron: Honoraria; Jazz: Honoraria, Speakers Bureau; BMS: Honoraria, Speakers Bureau; Agios: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal